Clicking molecules
This year's Nobel Prize in chemistry went to the scientists who developed click chemistry, a technique that quickly and easily bonds molecules together. For professor of bio-organic synthesis Hermen Overkleeft (53), this Nobel Prize did not come as a surprise.
Anyone who wants to study living cells faces a tough job. You can't just use any other chemical, because there's a good chance that it will interfere with the biological system. "The click reaction is now optimized in such a way that it does not affect living cells. You can think of it as the clasp of a beaded necklace. The two parts of the clasp can click together perfectly while not affecting the rest of the chain."
"With this, the researchers have introduced a whole new way of thinking. They have found a method of trying to understand or influence biological systems by adding something not biological to them. When I first read the article from the year 2000, I thought, well, I would have liked to have come up with this myself. I knew immediately that this was a visionary method that would completely change the field."
"And it has," laughs Overkleeft. "There is already a Dutch company using click chemistry to make cancer medication." This company uses proteins that specifically bind to cancer cells. "With click chemistry, they link a very toxic substance to these proteins. In the patient, the proteins target tumor cells. Those cells then die because of the toxic substance attached to them."
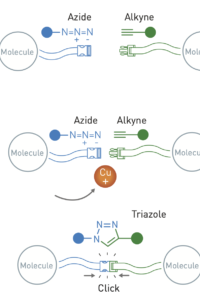
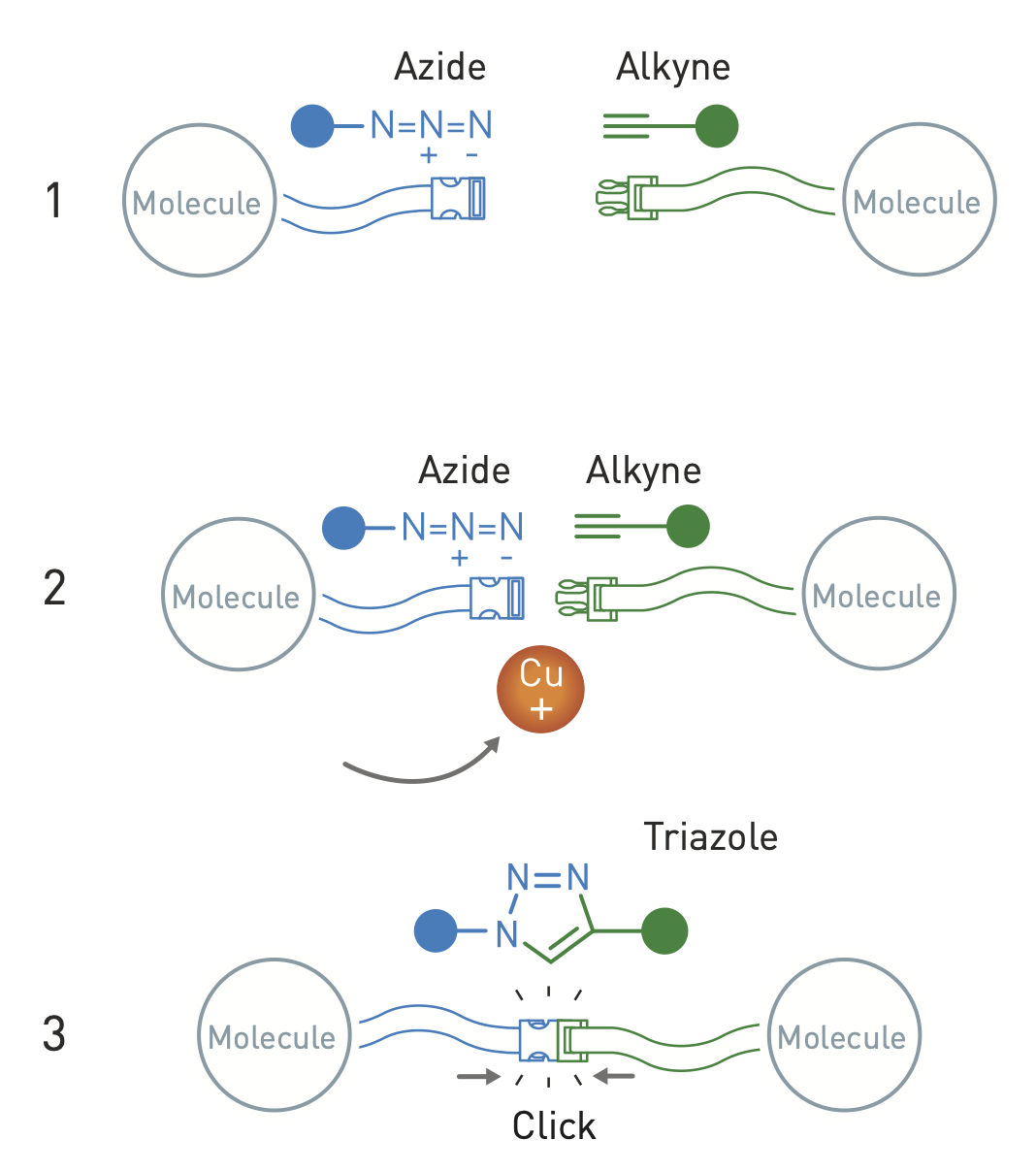
Blueprint
Overkleeft himself uses click chemistry to create inhibitors for proteins that promote a chemical reaction. Such proteins are called enzymes. "In my research, I make enzymes visible. To do this, I use click chemistry to make an inhibitor with a color on it. If the inhibitor binds to an enzyme, it is visible through the color. So I also use selective chemistry in living cells."
It was a celebration in the lab when Overkleeft first made a properly working inhibitor 15 years ago. "It was very challenging to put the molecule together. We worked for years before we had a good method to make the molecule. Then the inhibitor turned out to work even better than expected. We were able to stain enzymes very selectively. Even in laboratory animals. Of course, that was heartening for the research I was doing at the time. But the best part was that we had a good blueprint for other inhibitors."
The inhibitors are now being used to research new drugs. "I can treat a tissue or a laboratory animal with a candidate drug and my inhibitor. Then it becomes clear whether the drug hits the right enzymes," Overkleeft explains. "The inhibitor itself can also be transformed into a potential drug."
Furthermore, the inhibitors can help diagnose certain diseases. Some diseases are caused by a deficiency in a particular enzyme. Overkleeft's inhibitors can make these enzymes visible. "So you can see how big an enzyme deficiency patients suffer from," he said. And that's not all. "You can also see how well a therapy is working. After all, many therapies are aimed at increasing enzyme activity."
Craft
"But," Overkleeft adds, "I don't just make a molecule for every biologist who wants one. It has to be within our expertise. We mainly deal with protein and sugar structures. The biologists are also always involved in the process. We will always point out the difficulties to them. We never promise that the molecule will be ready for them in a few months."
Overkleeft himself is no longer bent over the test tubes daily to brew molecules. "That's because I spend a lot of time teaching, requesting research and writing articles," Overkleeft says. His workplace is now an office. "I do regret not being in the lab anymore, but besides being a science lab work is also a craft. You can only do the work well if you have routine. Well, I lost that, of course."
The hands-on work is done by the doctoral students and postdocs in Overkleeft's research group. "Doing research is a group effort. Wherever I said 'I,' it's 'we.' We make sure a lot of people work together."
That collaboration is typical throughout the field. "In organic chemistry and biochemistry, there is a lot of collaboration. Starting materials are made together or exchanged with each other. People learn from each other. In the end, that's also what got the Nobel Prize."





0 Comments
Add a comment