Human Gene Editing: Science (Non-)Fiction?
Human gene editing has long been a popular subject in science fiction. Some of these stories, such as GATTACA and The Island of Dr. Moreau, envisioned dystopian worlds ruled by social inequality, genetic mishaps and discrimination.
Others, such as Elysium and Deadpool, also portrayed the transformative promise of genetic engineering to enhance human potential and eliminate disease. Today, these once-fictional ideas are edging closer to reality, as the first medical applications of human gene editing are already reaching the clinic. Still, the question remains: will this real-world story have a similar transformative plot?
Modern Gene Editing: CRISPR/Cas9
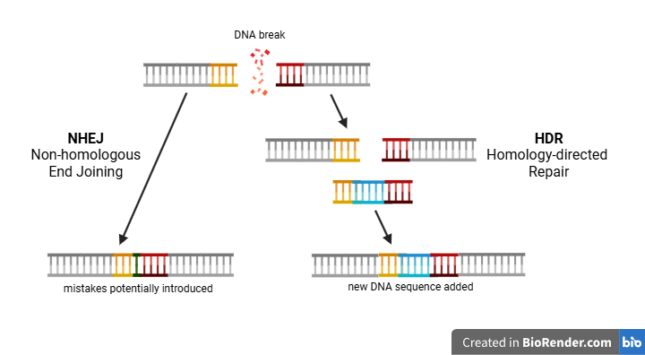
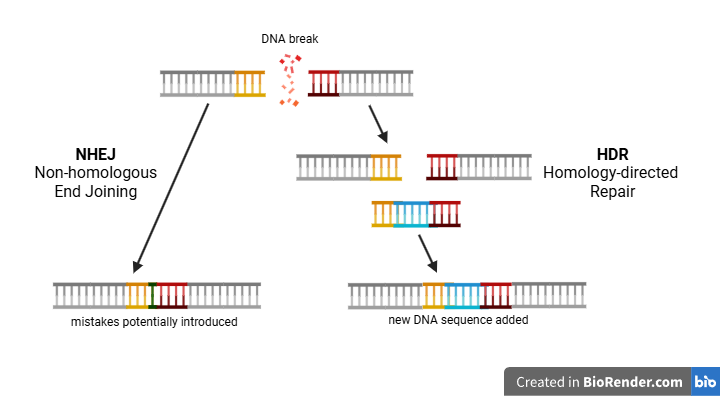
The story of modern gene editing applications started in 2012 with the discovery of CRISPR/Cas9. This RNA-protein complex is a naturally occurring defense system found in certain bacteria and archaea, but researchers have repurposed it as a tool for gene editing. It is often illustrated as a pair of molecular scissors, as it can ‘cut’ DNA by introducing targeted double-stranded breaks in the genome. Once such a break occurs, the cells utilise multiple strategies to repair the DNA, each with different consequences for the genetic code.
To illustrate the two DNA repair strategies, imagine that a pair of scissors - CRISPR/Cas9 - is used to cut a page of a book into two parts. The simplest and fastest way of restoring the page is to immediately stitch both parts together without paying attention to the alignment of the sentences. This ‘quick-fix’ repair reflects the mechanism of Non-homologous end joining (NHEJ), in which the broken DNA ends are directly reattached. However, as the analogy suggests, the sentences in the book - the information in the DNA strand - might no longer be readable. Notably, the resulting DNA strand often contains errors or missing information, potentially disrupting the gene’s function.
The alternative, and more complex, strategy involves the precise aligning of the stitch by looking at the same page from another copy of the book. This ‘high-fidelity’ repair mirrors the mechanism of Homology-directed repair (HDR). After a DNA break occurs, HDR fills in the original sequence based on a similar DNA template. If such a template is supplied with a slightly altered sequence around the break, HDR will introduce these changes in the genetic material.
Current Clinical Treatments Using CRISPR/Cas9
Thus, after a CRISPR/Cas9-induced break, researchers can disrupt or edit genes by exploiting the cell’s ‘quick-fix’ NHEJ or the ‘high-fidelity’ HDR, respectively. While NHEJ can occur directly in the cells where CRISPR/Cas9 is active, HDR requires a DNA template. Since supplying such a DNA template to cells poses an additional challenge, the current clinical treatments using CRISPR/Cas9 exclusively rely on NHEJ.
Treatment of Sickle Cell Disease
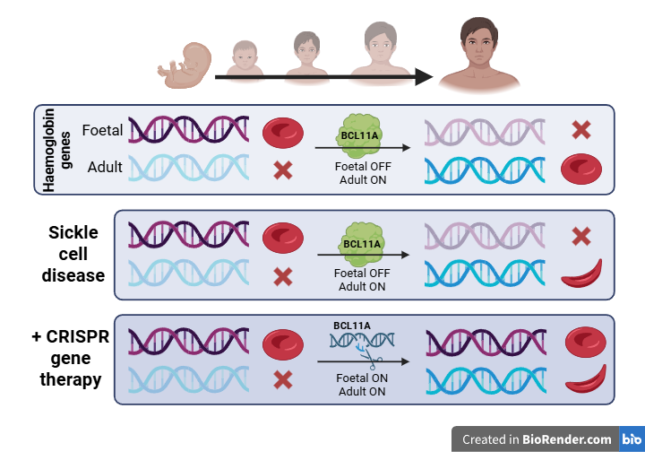
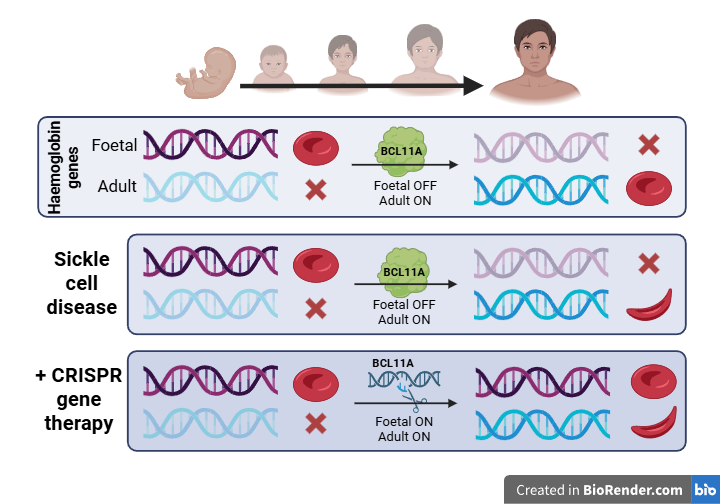
In 2023, just eleven years after the initial discovery, a CRISPR/Cas9-based therapy was approved for treatment of sickle cell disease (SCD). This disease is caused by a specific mutation in the β-globin gene, resulting in the production of the abnormal hemoglobin S (HbS). When oxygen levels are low, HbS aggregates and causes red blood cells to adopt an abnormal sickle shape. Compared to their healthy, disc-shaped counterparts, these red blood cells have a shorter life span, are less flexible and can block blood vessels. This leads to impaired oxygen provision, progressive organ damage and potential early death for individuals with the disease.
An obvious, but technically complex, approach to treat SCD would be to restore the mutation in the β-globin gene through HDR. However, for reasons outlined above, HDR remains difficult to apply efficiently in patients. Therefore, researchers opted to exploit the simpler NHEJ approach to restore the function of hemoglobin indirectly. Notably, red blood cells also contain a gene for foetal haemoglobin, which is usually inactivated several weeks after birth by another gene, BCL11A. By introducing targeted double-stranded breaks in BCL11A using CRISPR/Cas9, NHEJ can disrupt its sequence and effectively silence the gene. This silencing reactivates foetal haemoglobin production, which can be incorporated in red blood cells to restore their function.
For the red blood cells to incorporate the foetal haemoglobin, the editing with CRISPR/Cas9 must occur in their progenitor cells, the haematopoietic stem cells (HSC).
First, HSCs are isolated from the patient's bone marrow and CRISPR/Cas9 is subsequently applied. Treating the cells outside of the human body (‘ex vivo’) enables precise control and monitoring of the edited cells. The resulting HSCs are transplanted back into the patient, where they repopulate the bone marrow and begin producing healthy red blood cells. The described treatment, named Casgevy, showed great results in clinical trials, with the elimination of acute SCD-related symptoms in 97% of the patients.1
Treatment of Transthyretin Amyloidosis
Besides such ex vivo applications, CRISPR/Cas9 has also been applied inside the human body (‘in vivo’) to treat transthyretin amyloidosis (ATTR). ATTR is caused by mutations in the transthyretin (TTR) gene, leading to the production of misfolded TTR proteins. These misfolded proteins aggregate and accumulate in tissues, such as the heart and nerves, causing progressive organ damage.
The treatment of ATTR aims to reduce the production of TTR. To do this, CRISPR/Cas9 is delivered to the body to cause NHEJ-dependent gene disruption in the TTR gene. This therapy, known as NTLA-2001, is currently in the last stages of clinical trials and is the first example of an in vivo CRISPR/Cas9 treatment tested in humans. Early clinical trial results have shown that a single dose can reduce circulating TTR protein levels by over 80%, a dramatic decrease that is expected to slow or even halt disease progression.2
Future Perspectives on CRISPR/Cas9 Treatment
The existing clinical applications of CRISPR/Cas9 already demonstrate the promise of CRISPR/Cas9 as a transformative clinical tool. The next chapter of research is focussing on altering genetic codes with HDR - even close to home. A research group at the LUMC is attempting to utilise an HDR-dependent CRISPR/Cas9 technology for the treatment of severe combined immunodeficiency (SCID). Approximately 20% of SCID is caused by mutations in the RAG gene, which encodes an essential protein for the development of the immune-regulating T- and B-cells. Without functional T- and B-cells, patients suffer from a severely compromised immune system, often resulting in early mortality.
Potential Treatments Using HDR
In principle, the mutations underlying RAG-SCID could be corrected by a treatment with CRISPR/Cas9 and a DNA template with the corrected gene. In addition, treatment could occur ex vivo, similar to the therapy for sickle cell disease, since HSCs are also the progenitors for T- and B-cells. This complete strategy was tested in a pre-clinical trial with mouse models.3 After transplantation of edited HSCs, the researchers observed a restoration of RAG expression and functionality, leading to a successful restoration of T- and B-cell development. Thus, this study represents a proof-of-concept of using HDR-correction for the treatment of RAG-SCID, although a lot more effort is required to make the treatment available for humans.
The emerging realisation of this early HDR-based CRISPR/Cas9 approach sets the stage for the next chapter in the story: a future where rewriting genetic codes has the potential to revolutionise the treatment of a broad range of genetic disorders.4 Examples of such disorders include (but are not limited to): Duchenne Muscular Dystrophy, Huntington’s Disease, Cystic Fibrosis, Gaucher Disease, and Amyotrophic Lateral Sclerosis (ALS).
Remaining Challenges for CRISPR/Cas9 Treatments
However, the fact that these treatments are not yet realised in the clinic highlights the practical challenges that remain. These include designing safe and efficient delivery methods of CRISPR/Cas9, unwanted off-target effects, and countering immune activation.5 Beyond these technical hurdles, a variety of ethical boundaries cannot be overlooked. For one, editing of germline cells - which pass on their genes to the next generation - is still very much controversial, as this will irreversibly spread the genomic alterations among humanity. Secondly, clear boundaries are required to prevent the use of CRISPR/Cas9 for dangerous and non-essential applications. Finally, given the exceptional costs of the technique, considerations of social equity are essential to avoid the dystopian inequalities cautioned in science fiction.
Conclusion
Through the adoption of CRISPR/Cas9, gene editing of humans has moved from science fiction to early medical reality. Initial clinical successes, such as the treatments for SCD and ATTR, already demonstrate the transformative potential. In the future, the efforts towards HDR-dependent editing could expand the scope of CRISPR/Cas9 applications to a wide range of hereditary disorders, offering hope for conditions that were previously untreatable. Thus, the story of CRISPR/Cas9 is still far from over and this leaves us on the edge of an unfolding cliffhanger.
Sources
Frangoul, H., Locatelli, F., Sharma, A., Bhatia, M., Mapara, M., Molinari, L., Wall, D., Liem, R. I., Telfer, P., Shah, A. J., Cavazzana, M., Corbacioglu, S., Rondelli, D., Meisel, R., Dedeken, L., Lobitz, S., De Montalembert, M., Steinberg, M. H., Walters, M. C., . . . Grupp, S. A. (2024). Exagamglogene Autotemcel for Severe Sickle Cell Disease. New England Journal Of Medicine, 390(18), 1649–1662. https://doi.org/10.1056/nejmoa...
Gillmore, J. D., Gane, E., Taubel, J., Kao, J., Fontana, M., Maitland, M. L., Seitzer, J., O’Connell, D., Walsh, K. R., Wood, K., Phillips, J., Xu, Y., Amaral, A., Boyd, A. P., Cehelsky, J. E., McKee, M. D., Schiermeier, A., Harari, O., Murphy, A., . . . Lebwohl, D. (2021). CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. New England Journal Of Medicine, 385(6), 493–502. https://doi.org/10.1056/nejmoa...
Castiello, M. C., Brandas, C., Ferrari, S., Porcellini, S., Sacchetti, N., Canarutto, D., Draghici, E., Merelli, I., Barcella, M., Pelosi, G., Vavassori, V., Varesi, A., Jacob, A., Scala, S., Ricci, L. B., Paulis, M., Strina, D., Di Verniere, M., Sergi, L. S., . . . Villa, A. (2024). Exonic knockout and knockin gene editing in hematopoietic stem and progenitor cells rescues RAG1 immunodeficiency. Science Translational Medicine, 16(733). https://doi.org/10.1126/scitra...
Knott, G. J., & Doudna, J. A. (2018). CRISPR-Cas guides the future of genetic engineering. Science, 361(6405), 866–869. https://doi.org/10.1126/scienc...
Uddin, F., Rudin, C. M., & Sen, T. (2020). CRISPR gene therapy: applications, limitations, and implications for the future. Frontiers in Oncology, 10. https://doi.org/10.3389/fonc.2020.01387






0 Comments
Add a comment