Four physics facts to change how you view the world
Physics is incredibly cool, but it’s also hard. Exposing the universe’s secrets takes a good dose of dedication, endurance and mathematics. Good news: I studied physics so you don’t have to. Here are four physics facts to change how you view the world. Without maths.
1. Black holes
Let’s start easy: black holes! These humongous stellar objects certainly need a two-seater on the list of life-changing physics. Black holes are intrinsically mind-boggling, in both the fascinating and the terrifying kind of way. For anyone who’s never heard of them (in which case, where have you been?), here’s a brief explanation.
Every planet has its own escape velocity, which is the speed you need to escape its gravitational pull. Earth, for example, has an escape velocity of 11 kilometers per second. Imagine you’re throwing a ball into the air: after a while it falls back to earth. However, if you were to throw a ball at 11 kilometers per second, it would escape earth’s gravity and never fall back down. It would float away into the universe like a loose helium balloon (that’s going 39,600 km/h).
The more massive an object is, the greater its escape velocity. So, Einstein theorized, there must be an object so dense, so heavy, so stupendously massive, that its escape velocity is more than the speed of light: 1,000,000,000 km/h. As it turns out, Einstein was correct (as always): these stupendous objects exist! Being so dense that not even light can escape, they remain in the elusive shadows: black holes.
An interesting fact about black holes is that they have no physical surface. Planets, moons, stars and other celestial objects have a surface: you can see where the object begins and ends. Black hole images appear very similar (it’s still a spherical object, just black), but in reality you’re looking at something fundamentally different. The black sphere you see in images, is an optical border: on the outside light can still escape, on the inside it cannot. It is not a physical surface. The actual mass is buried deep within the sphere, contained in an infinitesimally small volume called a singularity. So black holes aren’t just very dense, they are infinitely dense.
This is why some people like to call black holes ‘space vacuums’, sucking up everything around them. And although that’s a nice and terrifying analogy, I find it a pretty sucky explanation.
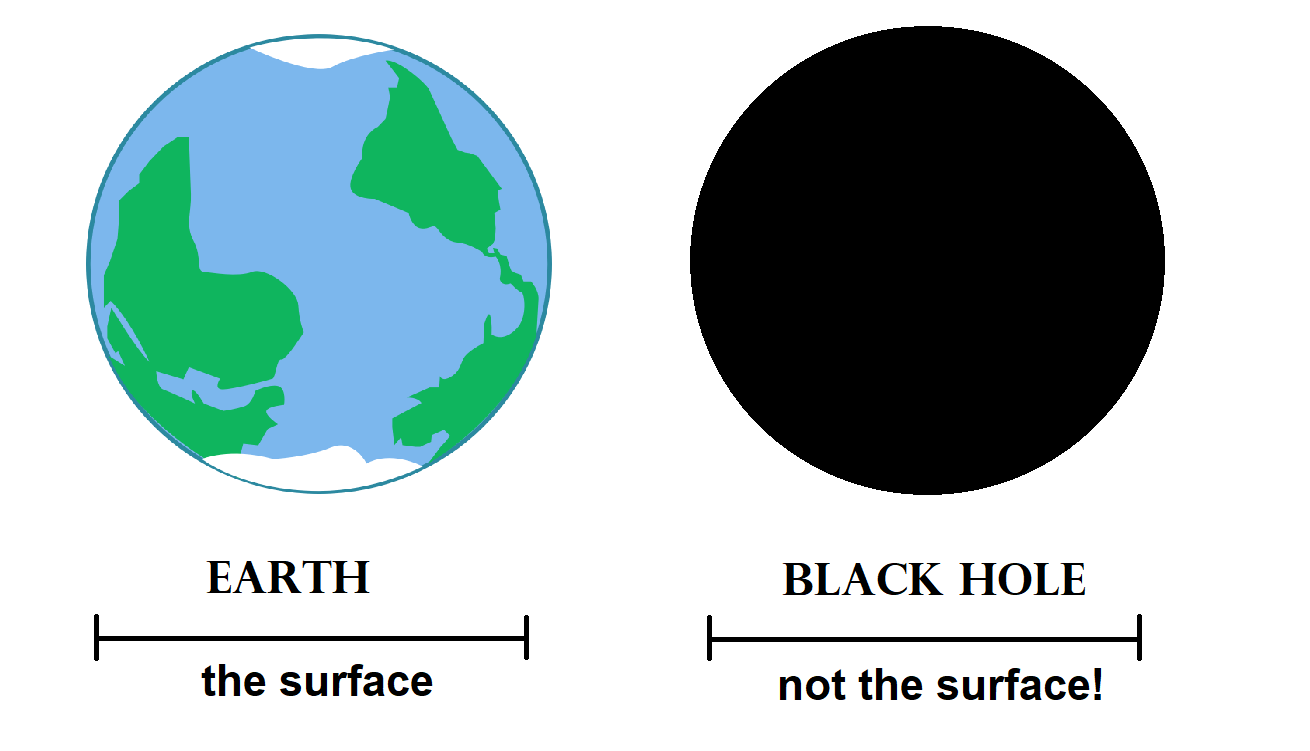
Although Earth and a black hole may appear similar in shape, they are fundamentally different. Image: Serafine Beugelink
2. Atoms
Now let’s dive from astronomically big to fundamentally small: atoms. What’s inside an atom? Protons, neutrons, electrons? Well yes, but no. Atoms primarily consist of nothing. Emptiness. Take hydrogen for example: the nucleus fits into the atom 5,000,000,000,000,000 times! To put this into perspective: if the atom were a cathedral, the nucleus would be a fruit fly.
So then what is this emptiness in between? If you said air, go directly to jail. Although air may appear ‘empty’, it is completely filled with atoms (like oxygen). The emptiness in atoms is just that: empty. It’s a vacuum. Thus, everything around us, our entire physical world, contains more vacuum than particles. Way more.
3. Entropy
Entropy is a term you might have heard before. It’s often used as a synonym for chaos: the more chaotic a system is, the higher its entropy. Although that is true, the official definition of entropy sounds less sensational. It’s the number of microstates corresponding to a macrostate. Bear with me.
A macrostate is a macroscopic, birds-eye view of a situation. For example, “a messy bedroom” is a macrostate. A microstate is the microscopic, detailed view of that situation. Your bedroom can be messy because the bed isn’t made up, or because your socks are on the floor, or because your clothes are sprawled everywhere... These are all different microstates, resulting in the same macrostate: a messy room. Because this macrostate has so many possible microstates, it has a high entropy. Conclusion: a messy room has a high entropy.
Now let’s tidy up a bit: consider a neat and tidy bedroom. In this case, there are some restrictions. Your socks and clothes can no longer be anywhere, they’re confined (to the wardrobe or laundry basket, for example). The macrostate “a tidy bedroom” thus has less options (so less microstates) than “a messy room”. Conclusion: a tidy room has a low entropy.
This is where it gets interesting. The second law of thermodynamics says that the entropy of a system will increase over time. What does this mean for our bedroom? A tidy room will naturally get messy over time! So next time you feel bad about the mess in your room, just remember: it’s not your fault. It’s literally a law of nature.

A messy room has a higher entropy than a tidy room. Image: Serafine Beugelink / Unsplash
4. The definition of life
Now that we have a good foundation in entropy, we can use it to get philosophical. What’s the definition of life? A biologist would give a list of properties: it eats, it grows, it reproduces… But what about physicists? What do they consider to be the definition of life? Fortunately for us, such a definition exists. It was given by a certain physicist often associated with a cat: Erwin Schrödinger. According to Schrödinger, “life uses free energy to decrease or keep constant its entropy.” What does that mean?
Let’s look back at our messy versus tidy bedroom scenario. Over time, the room will naturally go from tidy to messy (increase in entropy). You could also go the other way: from messy to tidy (decrease in entropy). But as you can imagine, that would take work. Bedrooms get messy on their own, but they’ll never become tidy on their own: that takes work, actively tidying up. If you wanted to “decrease or keep constant” the entropy of your room, you would have to constantly do work. Picking up socks, wiping off dust, cleaning the floor… Making sure it stays tidy and the entropy stays low. And that is basically what life forms do internally.
A living body is actively tidying up inside, keeping its entropy low. Take your body’s genome for example. That’s where your DNA is constantly duplicated, and it’s normal that over time, errors can occur. Your enzymes are actively working: scanning the duplicated DNA, looking for mistakes, and then solving them. That would be the equivalent of someone coming into your bedroom, scanning it for messes and then tidying them. Thus keeping the room tidy and entropy low.
What if life didn’t “decrease or keep constant” its entropy? Well, then entropy would naturally increase over time. In other words: your body’s insides would get increasingly messy over time, accumulating more and more mistakes. Until your internal processes no longer function. You can imagine the consequences.
Clearly, it’s important for life to keep its entropy low. But we know that that takes energy (to tidy up). Where does life get this energy from? The answer is sunlight. Whether you get your energy from plants or animals, the underlying source is always sunlight. In fact, more than 99.999% of all life on earth gets its energy from the sun. Life uses sunlight to do work internally, to lower its entropy and keep itself alive.
Here’s another crazy thing to think about. This energy that we harvest from the sun, is the same energy that the sun is actively losing: radiating into the universe.
We can live because the sun is dying.






0 Comments
Add a comment