Magic with CRISPR-Cas: Conjuring a unicorn with DNA
Always wanted a unicorn? Or a luminescent bunny? To see if this is fact or fiction, editor Soraya Schachtschabel talks with the experts.
More and more has become possible in the world of science. An ape can play ping pong with its mind, a delicious steak can be made in-vitro, and cars can drive themselves. But can we use our knowledge to bring magic to life? In this article, we will see if we can create unicorns through gene editing.
Gene editing is a collective term for a myriad of microbiological techniques which alter DNA. Through these modifications properties of organisms can change, or new properties can appear. The newest mechanism in gene editing is CRISPR-Cas, a molecular pair of scissors which can cut DNA on the exact spot scientists want it to.
“CRISPR-Cas was originally a defense mechanism for bacteria against bacteriophages [viruses which attack bacteria, ed.]”, explains professor and head of Human Genetics at LUMC, Silvère van der Maarel. “The CRISPR-Cas system cuts DNA, but through modifications in the lab we made countless variants. By then fusing these variants with molecules with the functionality you’re looking for you can do all kinds of things with DNA.” This way, you can remove, add, disable, or enable a gene - a piece of DNA code. Sounds like the right technique to build a mythical being!
Magic Bullet?
In comparison to older gene editing techniques CRISPR-Cas has many advantages. “The variability makes it possible to do many different things, the precision is much higher than previous techniques and the selectivity of new CRISPR-Cas variants is getting better and better”, says Van der Maarel. Genetic modification is also much faster than in the past. “Previously we could be working on genetic modifications for years, but nowadays you can do it in a few weeks. If you want to change a gene you can just do it in a jiffy.”
The use of CRISPR-Cas already has many success stories. Last year, the first patient was treated in vivo for blindness by adding a single mutation in eye cells. CRISPR-Cas seems to be the solution for every genetic problem. “But”, argues Van der Maarel, “it’s no magic bullet, miracle drugs don’t exist. Even CRISPR-Cas can have problems with the specificity being too low, inefficiency of reaching target cells, or causing immune reactions, just like other methods.”
Mythical Design
But can we use CRISPR-Cas to make a unicorn? Van der Maarel is positive: “Yes, why not? You can combine a horse with a narwhal’s horn. But you have to understand that it’s more complex than you think. It’s not just turning on two genes and a horn appears on the horse’s forehead. There’s a great deal of complex developmental biology involved, with an intricate orchestration of multiple genes to create such a feature.”
Developmental biologist Maurijn van der Zee agrees. He usually works with insects, but for this query he is glad to take a detour to vertebrates. “It’s technically possible to make a unicorn, but you need to know how a horn develops in the original animal. For a narwhal, the horn is originally a tooth, so you would need to modify a tooth of a horse with the use of narwhal DNA. You could also use a rhinoceros horn.” The issue becomes that neither animal is a model organism and very little is known about their developmental tracks. “We don’t even know how a tooth develops in a mouse, the most well-known model organism, let alone in a narwhal.” In summary, we still don’t know enough about how horns grow in narwhals or rhinos to understand what we would need to change in a horse to have it grow a horn.
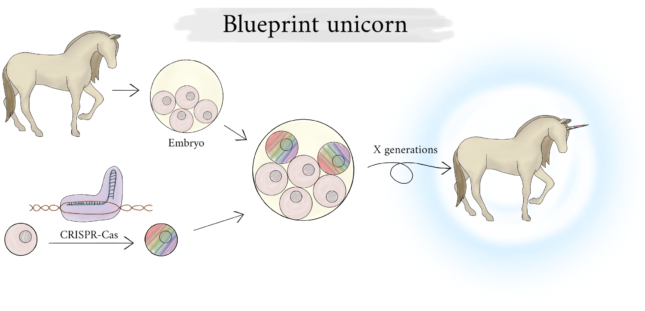
But once we have that all figured out, how would we turn horses into unicorns? “You really have to tinker with the foundation because you’re modifying developmental tracks. You can’t just change a few skin cells and suddenly a horn grows. The best idea is to add stem cells in the earliest stadium of an embryo”, Van der Zee tells us. A drawback is that you can’t do all modifications at once, it will only work after generations of horses. A lot of time is spent per generation. “See, with mice it already takes a PhD student two years to create a genetically modified variant, and mice have a very short generational turnover. Imagine how long it would take before you could have a unicorn.”
Illegal Unicorns
Other than the question of whether we can build a uniform, there is also the question of whether we want to or should. What use is a horn for a horse, and will there not be adverse side effects because we messed with their DNA? What is the use of creating unicorns, except being a special addition to a petting zoo? These are issues which society has to ponder, not just for questions like these but also slightly more serious ones.
Regulations-wise it is sadly currently impossible to create a unicorn. “In Europe, regulations are way behind what is actually possible. The rules are maybe 40 years old, while the methods and possibilities have completely changed”, finds university teacher Sylvia de Pater. She herself uses CRISPR-Cas in her research into DNA-recovery processes in plant cells. “I think the argument that you can see what the final product is - because you can read the genetic code of a genetically modified organism from front to back - is a good argument that the regulation should be changed. But I’m not saying it should be completely open, because then you would get people doing weird stuff.” Like making a unicorn.
For now, it seems impossible to conjure a unicorn with CRISPR-Cas, but maybe in the coming years we will learn more about developmental tracks and the law allows us to do more than we currently can. Who knows, perhaps in a few centuries these mythical beings can be found in a futuristic petting zoo.
Extra: CRISPR-Cas research in labs in Leiden
Silvère van der Maarel, LUMC
For more than twenty
years Van der Maarel has been doing research into the incurable muscle
dystrophy FSHD. This affliction causes the transcription factor DUX4 to
be switched on while it is not supposed to be. A transcription factor
causes other genes to activate. In this case, DUX4 causes severe muscle
weakness and atrophy. To see whether CRISPR-Cas can help in treating
this disease, Van der Maarel and his team grew cells from FSHD-patients
and corrected the DNA mutation specific for FSHD with the use of
CRISPR-Cas. Thanks to this, the modified cells produced much less DUX4,
which is a good result for hopeful patients.
Sylvia de Pater, IBL
Just like CRISPR-Cas has
been copied from a bacterial system, De Pater is also working with a DNA
system based on the survival mechanisms of bacteria. In this case, it
is the T-DNA of Agrobacterium. This bacterium puts a bit of its
own DNA into a plant cell which makes the plant produce opines,
products which only this specific bacteria can use. There are also genes
on the T-DNA for plant hormones, which create a tumor on the plant that
produces a lot of opines. This way, the bacteria are participating in
agriculture.
De Pater is looking into how the integration of T-DNA into the plant genome works, and how this system can be optimized to use in the plant industry to create transgenic plants. She focuses on recovery routes of double-strand breaks in the DNA and she investigates these routes in various mutants of the Arabidopsis plant by making breaks in the DNA with CRISPR-Cas.
Maurijn van der Zee, IBL
For his research into
the development of mealworm beetles Van der Zee compared DNA of two
groups of beetles to each other. One group develops fast while in their
egg, while the other develops slowly. It turns out the difference is
caused by a change on a single point in the hormone ecdysone. This
protein has a role in the formation of the pupa of insects, but could
also have a role in the development of the egg. Van der Zee’s research
is looking into mimicking the mutation with CRISPR-Cas. Discovering how
an insect can regulate its development and the timing of that
development can tell us a lot about how they can adjust themselves to
changes in their environment due to global warming.





0 Comments
Add a comment